On Aug. 14, 2023, U.S. Food and Drug Administration (FDA or the Agency) Commissioner Robert Califf provided an update on changes to the Agency’s dietary supplement program, under the proposed human foods program redesign. In January 2023, the FDA announced it would transform the current organizational structure of the food programs into one unified Human Foods Program (HFP). The FDA has now executed on the reorganization and has provided further guidance on how the new structure will impact the Agency’s oversight of human foods, including dietary supplements.
As described further below, the new structure significantly reorganizes FDA’s current approach to human foods regulatory oversight, such that all existing programs will now sit under the unified HFP. To better illustrate the program updates, FDA issued the graphic below highlighting the new unified HFP and how the existing programs transition into the new model:
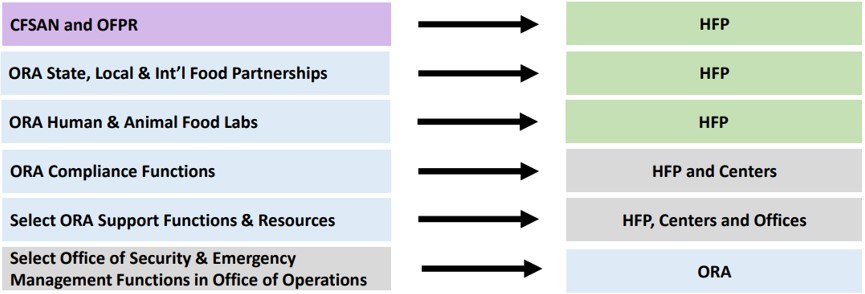

In the Aug. 14 announcement, Commissioner Califf also addressed:
- The establishment of the new Office of Food Chemical Safety, Dietary Supplements, and Innovation;
- The Agency’s enhancements to its risk management framework across the foods portfolio; and
- The development of new surveillance methods and tools aimed at bolstering the Agency’s oversight of dietary supplements.
.
Changes to the Office of Food Chemical Safety, Dietary Supplements, and Innovation
On Jan. 31, 2023, Commissioner Califf announced a proposed redesign of the FDA’s human foods program. The stated purpose of the redesign was to respond to findings from an external evaluation by the Reagan-Udall Foundation, as well as a separate internal review of the Agency’s response to significant issues in the infant formula supply chain. The findings from the reviews included recommendations for improvement, including modernizing data systems, improving emergency response systems, and building a more robust regulatory program.
As part of the redesign, the functions of the Center for Food Safety and Applied Nutrition (CFSAN), Office of Food Policy and Response (OFPR), and certain functions of the Office of Regulatory Affairs (ORA) are unifying into the new HFP. Additionally, under the new organizational structure, the Office of Dietary Supplement Programs (ODSP) will report to the new Office of Food Chemical Safety, Dietary Supplements, and Innovation (OFCSDSI). Under the proposed structure, the OFCSDSI director will report to a deputy commissioner.
In his statement, Commissioner Califf reiterated that currently there are no plans to reduce ODSP’s resources, and it will remain the lead office responsible for overseeing the Agency’s responsibilities under the Federal Food, Drug, and Cosmetic Act (FD&C Act), as amended by the Dietary Supplement Health and Education Act (DSHEA).
Updated Risk Management Framework
Commissioner Califf also highlighted that the unified HFP should help facilitate a more effective risk-management framework across the foods program, and that HFP and ORA should work in tandem to share resources and coordinate regulatory oversight, including surveillance efforts and safety assessments. By working under an aligned management framework, the offices should be better equipped to identify and respond to public health risks. Commissioner Califf also noted that ODSP is responsible for ensuring that risks related to dietary supplements are appropriately assessed and managed with the Agency’s established oversight tools.
Updated Surveillance Methods and Tools
The Agency also intends to develop new surveillance methods and tools to better identify and respond to public health threats as early as possible. Commissioner Califf specifically noted the importance of having access to all available data about dietary supplements and ensuring that the Agency has the necessary resources and tools to sustain the dietary supplement program as a high-priority area. Key areas of focus will be “leveraging modern computational, analytical, toxicology and research methods and tools across the HFP” to help enhance oversight of the dietary supplement program.
Looking Ahead
Commissioner Califf indicated he would be sharing additional information later this year about how the dietary supplement program would be positioned within the HFP. Based on the information already shared, the Agency is prioritizing oversight of dietary supplements and will be bolstering efforts to identify dietary supplement companies that fail to meet regulatory requirements, including those concerning safety, labeling, and claims. Companies involved in manufacturing, distributing, marketing and selling dietary supplement should take note of the expected increased scrutiny and consider comprehensive compliance assessments to address identified gaps.


